http://hyperphysics.phy-astr.gsu.edu/hbase/chemical/bond.html
http://www.visionlearning.com/en/library/Chemistry/1/Chemical-Bonding/55
http://www.chem1.com/acad/webtext/chembond/
http://www.chem4kids.com/files/atom_bonds.html
http://www.chem4kids.com/
http://www.acs.org/content/acs/en.html
http://www.rsc.org/
http://chemistry.about.com/
https://chemistry.stanford.edu/
http://www.sciencedirect.com/science/journal/03088146
http://www.nature.com/nchem/index.html
http://scienceworld.wolfram.com/chemistry/
http://chemistry.bd.psu.edu/jircitano/gases.html
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch4/gaslaws3.html
http://antoine.frostburg.edu/chem/senese/101/gases/
https://www.khanacademy.org/science/chemistry/gases-and-kinetic-molecular-theory/ideal-gas-laws/v/ideal-gas-equation-pv-nrt
Tuesday, May 10, 2016
Bonding 3
In general, strong chemical bonding is associated with the sharing or transfer of electrons between the participating atoms. The atoms in molecules, crystals, metals and diatomic gases—indeed most of the physical environment around us—are held together by chemical bonds, which dictate the structure and the bulk properties of matter.
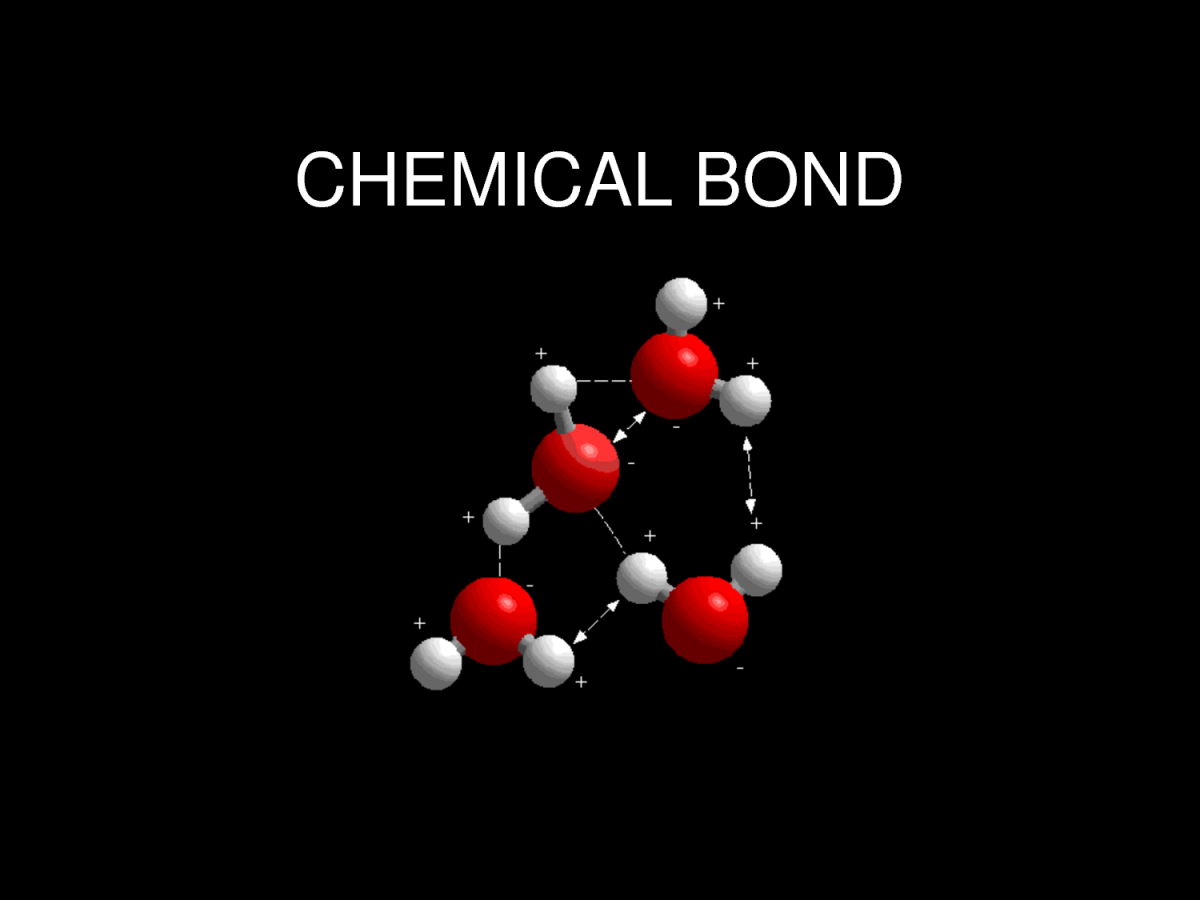

Bonding 2
Since opposite charges attract via a simple electromagnetic force, the negatively charged electrons that are orbiting the nucleus and the positively charged protons in the nucleus attract each other. An electron positioned between two nuclei will be attracted to both of them, and the nuclei will be attracted toward electrons in this position. This attraction constitutes the chemical bond. Due to the matter wave nature of electrons and their smaller mass, they must occupy a much larger amount of volume compared with the nuclei, and this volume occupied by the electrons keeps the atomic nuclei relatively far apart, as compared with the size of the nuclei themselves. This phenomenon limits the distance between nuclei and atoms in a bond.


Bonds 1
A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between atoms with opposite charges, or through the sharing of electrons as in the covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" such as covalent or ionic bonds and "weak bonds" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.

Monday, May 9, 2016
Energy and phase changes 2
The unit most associated with energy is joules, but we can use the unit calorie when we are taking about the amount of energy to raise the temperature of one gram of substance by 1C.
Intermolecular-occurs between molecules.
Intramolecular-occurs within a molecule.
Energy and phase changes
Chemical reactions involve not just the conversion of reactants into products, but also involve an energy change in the form of heat. Heat released as the result of a reaction, or heat abosorbed as a reaction proceeds. Energy changes happen when the chemical bonds are moved around.
There are four types of energy. 1)radiant energy: energy from the sun or solar energy. 2)thermal energy: energy associated with the random motion of atoms. 3)chemical energy: energy stored within the structural units of chemical substances. 4) potential energy: energy stored in the composition of a substance.
Thursday, May 5, 2016
Biodiesel 3
The main ingredient in biodiesel is methanol. Biodiesel is commonly produced by the transesterification of the vegetable oil or animal fat feedstock. There are several methods for carrying out this transesterification reaction including the common batch process, supercritical processes, ultrasonic methods, and even microwave methods.
Biodiesel 2
Biodiesel is meant to be used in standard diesel engines and is thus distinct from the vegetable and waste oils used to fuel converted diesel engines. Biodiesel can be used alone, or blended with petrodiesel in any proportions.
Biodiesel 1
Biodiesel is a clean source of energy that should be used more often. It releases less harmful chemicals than other forms of fuel and is also renewable. We get it from breaking down used vegetable oil and processing it into a usable form of energy.
Tuesday, May 3, 2016
Charles' Law
Charles' Law tells us that temperature and volume vary directly with each other as long as the pressure is constant. This does not apply for absolute zero, which is a theoretical temperature in which all movement stops. Including the movment of electrons.
Kelvin
The temperature for all Charles' Law problems must be done in kalvin. The Kelvin scale is an absolute scale. The conversion for kalvin is: C+273.15
Gas law 2
Boyle's Law uses pressure and volume to find out curtain aspects about a gas. It tells us that the realationship between pressure and volume is an inverse relationship. This holds true at a constant temperature.
Gas law 1
Gases form homogeneous mixtures. Also they are highly compressible and fill what ever they are in. The collisions that take place between the molecules are elastic. A barometer is commonly used to measure atmospheric pressure.
Wednesday, April 6, 2016
bond polarity
 In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in electronegativity between atoms in a compound and the asymmetry of the compound's structure. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points.
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Molecular polarity is dependent on the difference in electronegativity between atoms in a compound and the asymmetry of the compound's structure. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points.
single and double bonds
A single bond between two atoms corresponds to the sharing of one pair of electrons. The electron density of these two bonding electrons is concentrated in the region between the two atoms, which is the defining quality of a sigma bond.
A double bond between two atoms is formed by the sharing of two pairs of electrons, one in a sigma bond and one in a pi bond, with electron density concentrated on two opposite sides of the internuclear axis. A triple bond consists of three shared electron pairs, forming one sigma and two pi bonds.
Quadruple and higher bonds are very rare and occur only between certain transition metal atoms.
A double bond between two atoms is formed by the sharing of two pairs of electrons, one in a sigma bond and one in a pi bond, with electron density concentrated on two opposite sides of the internuclear axis. A triple bond consists of three shared electron pairs, forming one sigma and two pi bonds.
Quadruple and higher bonds are very rare and occur only between certain transition metal atoms.

bonds beginning
A chemical bond is a lasting attraction between atoms that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between atoms with opposite charges, or through the sharing of electrons as in the covalent bonds. The strength of chemical bonds varies considerably; there are "strong bonds" such as covalent or ionic bonds and "weak bonds" such as Dipole-dipole interaction, the London dispersion force and hydrogen bonding.

Wednesday, March 30, 2016
Boidiesel
Today we learned about how to make biodiesel and how it effects our environment about us. We are also starting our work on our biodiesel video in which we present biodiesel as a good clean source of energy.
Wednesday, March 9, 2016
Trends in Electronegativity
Electronegativity of an element is defined to be the tendency of an atom to draw electrons tward itself when chemically combined with another element.


Trends in ionic size
The periodic table is made in a fashion that allows us to be able to easily guess the size of an atom compared to the other elements. This is the trend associated with the periodic table.
Energy level diagrams
Think of an electron configuration for a particular element like an address. This will make it easier to understand.
Orbitals
Today we learned about orbitals and how they pertain to the periodic table. Each principle energy level has its own type of shape for its orbital.
Periodic table activity
For this activity we had to use our knowledge of periodic trends to create our own periodic table from clues about unknown chemicals.
Spectroscopic analysis of Co and Cu ion
This lab was long and hard. It was time consuming because of all the writing we had to do for this lab. Also the machinery was difficult to figure out.
Acid base test
Today's test was one of the hardest tests we have taken all year. I studied for 5 days then the test date was moved back. Which was good because it gave me more time to study.
Acid-base reactions
These are the notes I took on our acid base reactions lecture. I have to say it was very difficult but I think I got it down.
Vitamin C lab
For this lab we had to determine the Vitamin C concentration in all of the juices we were provided. We did this by adding iodine to each solution drop by drop. Until the solution changed color. The hardest part of this lab was the math.
Molar mass of unknown acid
This lab was basically the same as the last titration lab we did. The only difference was that the acid was solid not liquid. This cause us to spend more time on the lab due to mixing the acid with water in order us to be able to tirate it.
Titration lab
This lab was fairly easy compared to most of the labs we have done in the past. First we needed a buret filled with .200M NaOH solution. Then we use the buret to titrate an avid and compare it to the unknown acid to determine the Molarity.
Sunday, February 21, 2016
Learning about electronic structures
Today we learned about wavelength and how it can tell the energy of an atom. The size of the wavelength gives us color if it falls into the spectrum that is visible to the human eye. We learned the two need to know equations C=Wf and E=hv. Using these equations we can even find the exact element that is burning.
Thursday, January 21, 2016
Wednesday, January 13, 2016
Detective lab
Today we did a lab to determine an unown chemical. In our story we need to determine our murder weapon and the Molarity of it. This would help us determine who the killer was in our story.
Tuesday, January 12, 2016
Molarity
Molarity is how scientists describe how concentrated a solution is. The formula for Molarity is: M=mol of solute
Subscribe to:
Posts (Atom)























